
Fitted with a variety of detectors, the laboratory essentially has three instrument techniques available, all of which are useful for both qualitative and quantitative analysis.
- Gas Chromatography (GC)
- Liquid Chromatography (HPLC)
- Thin Layer Chromatography (TLC) and Paper Chromatography
Read More
ICP-OES has become essential tool in meeting the new requirements for the ISO: 10993 Chemical Characterization and Leachates. We have extensive experience of developing and/or validating material specific methods to meet the new requirements.
- Inductively Coupled Plasma - Optical Emission Spectroscopy (ICP-OES)
- Atomic Absorption Spectroscopy (AAS)
- Fourier-transform infrared spectrometer (FTIR)
Read More


Training in both gravimetric and titrimetric techniques, together with colorimetry, is a requirement for all our analysts. This enables all them to perform a wide range of basic qualitative and quantitative tests such as those found in the Indian/British/United States/European/Japanese pharmacopoeias and other published methods, in addition to specific instrumental techniques.
When macro analysis (the determination of quantities of 0.1g or more) is being undertaken, these classical analytical chemistry techniques can often provide much better relative precisions than the more modern instrumental techniques.
Read More
The term "Phys/Chem testing" is taken from the GLP regulations and the OECD guidelines for the testing of chemicals, and refers to the determination of physical-chemical properties of materials, such as:
- Melting point / Melting range
- Boiling point / Boiling range
- Distillation range
- Viscosity of liquids
Read More

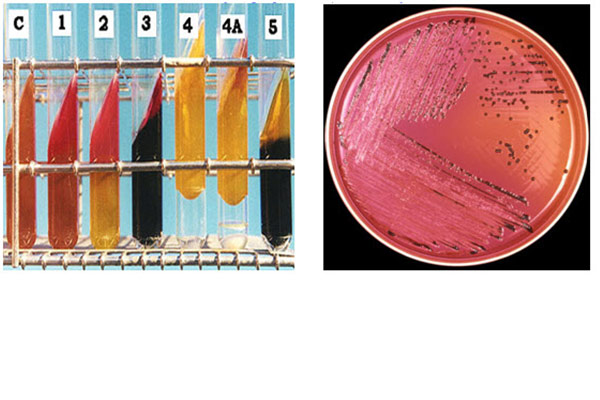
To ensure raw materials, finished products and production environments are safe from microbiological contamination.
Through our, highly experienced staff and extensive range of microbiological tests, SPARC provides customers with cost-effective quality assurance. We have a range of test methods available to meet your requirements.
Read More
Medical devices and their component materials may leach compounds or have surface characteristics that may produce undesirable effects when used clinically. The selection and evaluation of materials and devices intended for use in humans requires a structured program of assessment to establish biocompatibility and safety.
Current regulations, whether in accordance with the U.S. Food and Drug Administration (FDA), the International Organization for Standardization (ISO), require that manufacturers conduct adequate safety testing of their finished devices through pre-clinical and clinical phases as part of the regulatory clearance process.
Read More
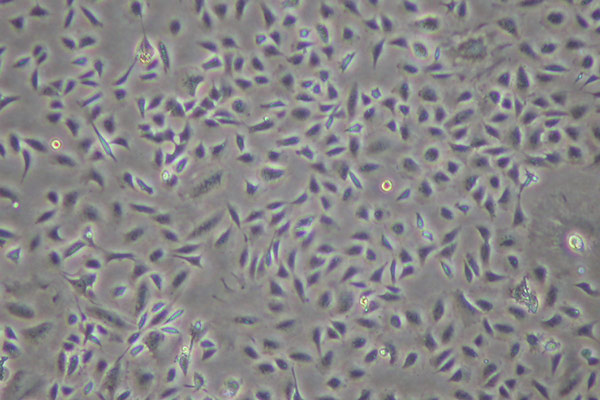
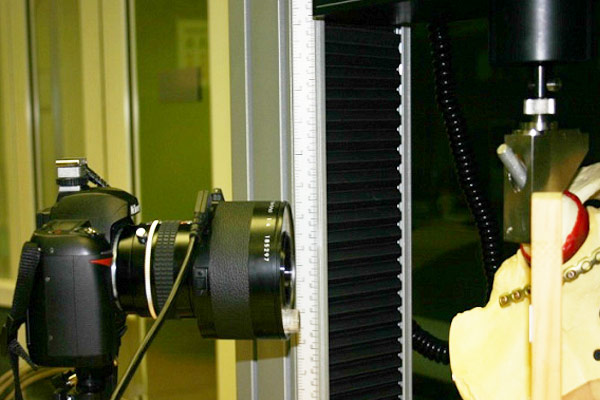
The laboratory carries out orthopedic biomechanical study.
The lab had conducted studies as per ASTM F382 / ASTM F384 / ASTM F543 and ASTM F1264. The current research field mainly includes the application of new orthopedic implants such as the customized prosthesis and fracture fixator, implant related biomechanical analysis of bone and cartilage.
Read More