Medical devices and their component materials may leach compounds or have surface characteristics that may produce undesirable effects when used clinically. The selection and evaluation of materials and devices intended for use in humans requires a structured program of assessment to establish biocompatibility and safety. Current regulations, whether in accordance with the U.S. Food and Drug Administration (FDA), the International Organization for Standardization (ISO), require that manufacturers conduct adequate safety testing of their finished devices through pre-clinical and clinical phases as part of the regulatory clearance process.
The guidelines provide a general testing framework to aid manufacturers in the assessment of device biocompatibility. The number and type of specific safety tests required to assess product safety and compliance are dependent on the individual characteristics of the device, its component materials, and its intended clinical use.
Within the general safety testing framework, it remains the responsibility of the device manufacturer to select and justify the specific tests most appropriate for the establishment of product safety and compliance with ISO and FDA requirements. It is recommended that testing be performed to comply with GLP regulations.
Choosing the correct path for biocompatibility testing has become more complex than ever. Having knowledgeable experts on your team to help develop complete submission packages is crucial to successfully moving your product to market.
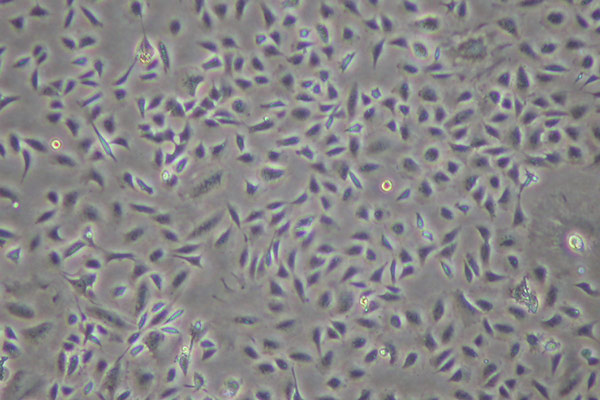
Cytotoxicity - ISO 10993-5
A cytotoxicity test determines whether a product or compound will have any toxic effect due to leachables on living cells. This test is generally used as a screening tool for raw materials or component products before they are put into the design of a medical device.
Sensitization – ISO 10993-10
Tests for sensitivity to any part of a device during exposure to the body for any length of time. This test is used to deteremine allergic or other sensitive reactions.
Irritation/Intracutaneous - ISO 10993-10
Determines how irritable a product or compound is to the body. Studies are made in combination with how the product or compound will be used and effected areas should be tested to determine the effect over time.
Acute Systemic Toxicity - ISO 10993-11
Identifies the effect of being exposed to a product or compound within 24 hours. Acute toxicity occurs after a single exposure or repeated exposures to the test subject. SubAcute symptoms appear within 14 to 28 days of delivery.
Subchronic Toxicity – ISO 10993-11
Studies that continue for 90 days or for up to 10% of a test subject’s life span are considered Subchronic. Studies that continue for longer than 10% of a test subjects life span are considered chronic.
Genotoxicity – ISO 10993-3
Genotoxicity, tests for gene mutations, changes in chromosomes, DNA and gene toxicities caused by products or compounds.
Implantation - ISO 10993-6
Studies the effects of products or compounds on living tissue. Exaggerated amounts of material should be used. It may be important to calculate the maximum amount of material that would be typically used and then implant multiples of that amount in an experiment.
Hemocompatibility - ISO 10993-4
Tests the effects of blood contacting the product or compounds on blood or blood components, directly or indirectly during routine use.
Chronic Toxicity - ISO 10993-11
Chronic toxicity studies can require that animal subjects be exposed to varying doses of test agents over an extended period of time.
Carcinogenicity - ISO 10993-3
This test should be performed only if there is data from other sources suggesting possible difficulties. This test is performed over the majority of the test subject’s life. It looks for tumorigenicity as well as chronic toxicity.
Reproductive/Developmental – ISO 10993-3
This study should only be performed when it is believed that the reproduction system will be affected by the product of compound. It tests the effects of the product or compound on the reproductive system, development of the embryo, as well as pre and postnatal development.
At our facilities in Delhi, we are able to undertake in-vitro biocompatibility testing of materials.
However, to enable us to provide complete spectrum of Biocompatibility testing studies, we have developed a network of reputable and reliable partner laboratories and collaborators, which provide specialized and niche services that greatly enhance our capabilities for solving your most complex problems.
Using the high ethical standards we hold ourselves accountable to,SPARC carefully selects partners who provide the highest quality results.
For more information on our Biocompatibility testing services you can get in touch using our Contact Form